Table of Contents - Why Low Hot Tub pH Matters and How to Restore Water Balance
- Understanding Hot Tub pH Balance
- The Critical Difference Between pH and Total Alkalinity
- Signs and Symptoms of Low pH in Your Spa
- Common Causes of Low pH in Hot Tubs
- Testing Your Water Before Adding Chemicals
- Chemical Solutions: Soda Ash vs. Baking Soda
- Step-by-Step Guide to Raising Hot Tub pH
- The Aeration Method: Raising pH Without Chemicals
- Managing pH in Different Hot Tub Types
- Troubleshooting Common pH Problems
- Prevention and Long-Term Maintenance
- Frequently Asked Questions About Raising Hot Tub pH
- Can I use baking soda to raise pH in my hot tub?
- How long do I have to wait to get in the hot tub after adding pH increaser?
- Is it safe to use a hot tub with low pH?
- What is the difference between pH Up and Alkalinity Up?
- Why does my hot tub pH keep dropping?
- Can I use vinegar to raise hot tub pH?
- How much pH increaser should I add to a 400-gallon hot tub?
This blog post may contain affiliate links. As an Amazon Associate I earn from qualifying purchases.
How to rise hot tub pH? The complete guide
Maintaining balanced water chemistry is one of the most critical responsibilities for any hot tub owner. Among all the chemical parameters you need to monitor, pH level stands out as the cornerstone of water quality and equipment longevity. When your hot tub’s pH drops too low, the water becomes acidic, creating a cascade of problems that affect everything from bather comfort to the structural integrity of your spa.
Understanding how to raise hot tub pH isn’t just about adding chemicals it’s about comprehending the underlying water chemistry, recognizing the warning signs of imbalance, and implementing sustainable maintenance practices. Whether you’re dealing with stinging eyes, corroding equipment, or water that just doesn’t feel right, learning to properly adjust pH will transform your hot tub experience from frustrating to enjoyable.
This comprehensive guide will walk you through everything you need to know about identifying low pH, understanding its causes, and implementing both chemical and natural solutions to restore proper balance. By the end, you’ll have the knowledge to maintain crystal-clear, comfortable water that protects both your investment and your health.
Understanding Hot Tub pH Balance
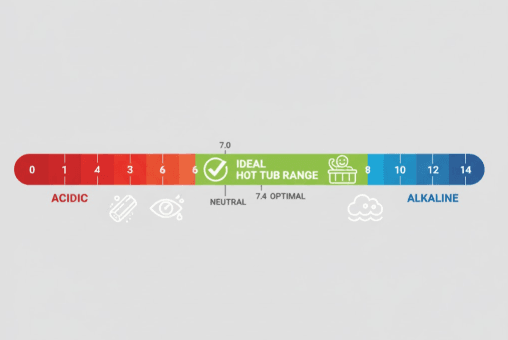
The term pH stands for “potential of hydrogen” and measures how acidic or alkaline your water is on a scale from 0 to 14. Pure water sits at neutral 7.0, while anything below 7.0 is acidic and anything above is alkaline (also called basic). For hot tub owners, this scientific measurement has very real, tangible consequences.
The ideal pH range for hot tub water falls between 7.2 and 7.8, with the sweet spot being 7.4 to 7.6. This narrow range isn’t arbitrary it’s been established through decades of research and practical experience to optimize sanitizer effectiveness, bather comfort, and equipment protection.
When pH drops below 7.2, your water enters the acidic zone where problems multiply rapidly. Acidic water becomes corrosive, eating away at metal components like heater elements, pump seals, and even the finish on your spa shell. The sanitizer you’ve added works inefficiently, burning off faster than it should and leaving your water vulnerable to bacteria and algae.
Human skin has a natural pH of around 5.5, but our eyes and mucous membranes are most comfortable in water that closely matches the pH of our tears approximately 7.4. This is why properly balanced hot tub water feels silky and comfortable, while acidic water causes irritation almost immediately.
For comprehensive guidance on maintaining optimal water chemistry, visitOne Hot Tub, where you’ll find expert resources for all aspects of spa ownership.
The Critical Difference Between pH and Total Alkalinity
Before you can successfully raise hot tub pH, you must understand the relationship betweenpH and Total Alkalinity. These two parameters work together like partners in a dance; you cannot manage one effectively without considering the other.
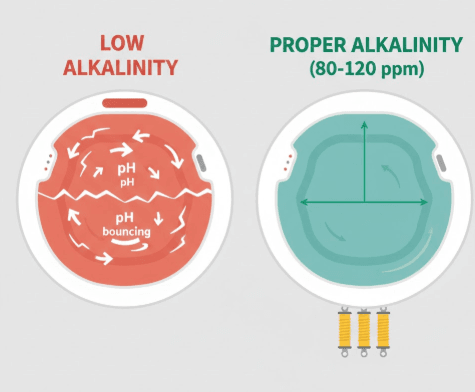
Total Alkalinity measures the concentration of alkaline substances in your water, primarily bicarbonates, carbonates, and hydroxides. Think of TA as your water’s resistance to pH changes a buffer that prevents wild pH swings when you add chemicals or when organic contaminants enter the water.
The ideal Total Alkalinity range for hot tubs is 80 to 120 parts per million (ppm), with 100 ppm being optimal for most situations. When your TA falls below this range, your pH becomes unstable and prone to what’s called “pH bounce” rapid, unpredictable fluctuations that make water balance nearly impossible to maintain.
Here’s the critical insight that many hot tub owners miss: if your Total Alkalinity is low, attempting to raise pH will provide only temporary relief. The pH will climb initially, then crash back down within hours or days because there’s insufficient alkaline buffer to hold it steady.
Conversely, when alkalinity is within the proper range, pH becomes much easier to adjust and maintain. The alkaline compounds in the water act like shock absorbers, cushioning the impact of environmental factors, bather load, and chemical additions.
This is why the golden rule of hot tub chemistry is to always adjust Total Alkalinity first, then fine-tune pH second. Trying to fix pH without first addressing alkalinity is like trying to steer a car with broken shock absorbers you might get where you’re going eventually, but the ride will be unpredictable and potentially damaging.
For detailed strategies on managing both parameters effectively, check out our guide on Hot Tub pH Control, which covers the full spectrum of water balance maintenance.
Signs and Symptoms of Low pH in Your Spa
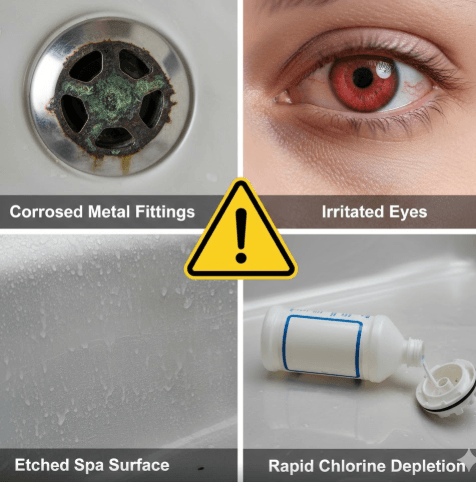
Recognizing the warning signs of low pH can help you address problems before they cause permanent damage. Acidic water presents itself through multiple channels visual, physical, and mechanical indicators that become increasingly obvious as the imbalance worsens.
Visually, low pH water often appears deceptively clear. Unlike high pH water that tends to become cloudy due to calcium precipitation, acidic water maintains its clarity even while causing significant damage. However, you may notice corrosion stains on metal fixtures, fittings, and jet faces telltale brown, green, or blue discoloration indicating metal dissolution.
The physical sensations are usually the first alerts most bathers notice. Acidic water causes eye irritation ranging from mild redness to intense burning. Your skin may feel itchy, dry, or uncomfortably tight after soaking. Some people experience respiratory irritation from chloramines that form more readily in low pH conditions, leading to a strong “chlorine smell” that’s actually a sign of improper water chemistry rather than too much sanitizer.
Equipment damage manifests gradually but relentlessly. Heater elements develop pinhole leaks or complete failures as the acidic water eats through the protective oxide layer. Pump seals deteriorate prematurely, leading to leaks and reduced circulation efficiency. The acrylic shell or vinyl liner may show signs of etching a dull, rough texture where the glossy finish once was.
Chemical inefficiency is perhaps the most frustrating symptom. Chlorine and bromine sanitizers work optimally within the 7.4 to 7.6 pH range. As pH drops, these sanitizers dissipate rapidly, forcing you to add more and more chemical to maintain proper levels. This creates a vicious cycle: more sanitizer usage leads to more pH suppression, which demands even more sanitizer.
Watch for these specific warning signs:
- Stinging or burning eyes within minutes of entering the water
- Skin that feels dry, tight, or itchy after soaking
- A strong chemical odor despite recently balanced chlorine levels
- Corrosion or discoloration on metal components
- Rapid chlorine or bromine depletion requiring frequent additions
- Wrinkled skin or fingernails that develop unusually quickly
- Equipment malfunctions, especially with the heater or pump
Early detection is your best defense. Testing your water at least twice weekly allows you to catch pH drift before it causes significant problems.
Common Causes of Low pH in Hot Tubs
Understanding why your pH drops helps you prevent future imbalances and address the root cause rather than just treating symptoms. Several factors can push your hot tub water toward the acidic end of the spectrum, often working in combination to accelerate the decline.
Heavy bather load is the most common culprit in residential hot tubs. Every person who enters your spa introduces organic compounds sweat, body oils, cosmetics, lotions, hair products, and skin cells. These contaminants consume sanitizer and release acidic byproducts as they break down. A hot tub party with multiple bathers can drop pH by 0.3 to 0.5 points in a single session.
Environmental factors play a significant role, especially for outdoor installations. Acid rain, which has a pH ranging from 4.0 to 5.5 depending on regional pollution levels, can gradually lower your spa’s pH. Leaves, pollen, dust, and other airborne debris that accumulate in uncovered tubs also contribute to acidification as they decompose.
Chemical usage patterns often inadvertently suppress pH. Trichlor chlorine tablets and bromine tablets are naturally acidic, with pH values around 3.0. While convenient for sanitization, regular use drives pH downward. Non-chlorine shock treatments based on potassium monopersulfate (MPS) are slightly acidic as well, causing gradual pH decline with each application.
Source water chemistry provides the foundation for your hot tub’s chemical behavior. If your municipal water supply or well water has naturally low pH or low alkalinity, you’re starting with a handicap. Some regions deliver tap water with pH as low as 6.5 and alkalinity under 40 ppm, making proper balance an ongoing challenge.
Water age and Total Dissolved Solids accumulation create long-term pH suppression. As months pass without a complete water change, dissolved minerals, spent chemicals, and organic residues accumulate. This “tired” water becomes increasingly resistant to chemical adjustments and tends to drift toward acidic conditions. High TDS water generally above 1,500 ppm should be drained and replaced regardless of other parameters.
Carbon dioxide absorption from the atmosphere represents a subtle but persistent factor. When water is agitated by jets and air bubblers, it releases excess CO2, which actually raises pH. However, when the water sits still in a covered spa, it can slowly absorb CO2 from trapped air, forming carbonic acid and lowering pH slightly.
Identifying which factors affect your specific situation allows you to implement targeted prevention strategies rather than constantly reacting to pH crashes.
Testing Your Water Before Adding Chemicals
Accurate testing is the foundation of effective water chemistry management. You cannot properly raise pH if you don’t know the current level or understand the complete chemical picture. Investing in quality testing equipment and developing good testing habits will save you time, money, and frustration.
Three primary testing methods are available to hot tub owners, each with distinct advantages and limitations. Test strips offer convenience and speed simply dip the strip in the water, wait fifteen seconds, and compare the color patches to the chart. However, accuracy can be questionable, especially as the strips age or if you misread subtle color differences.
Liquid drop test kits provide superior accuracy for critical parameters. These kits use reagent drops added to water samples, creating color changes that you compare to standardized charts. The FAS-DPD method for chlorine testing and phenol red for pH testing are industry standards. The primary disadvantages are the slightly more complex procedure and the need to replace reagents annually as they lose potency.
Digital pH meters deliver laboratory-grade precision, displaying exact pH readings to one or two decimal places. High-quality meters cost more initially but pay dividends in accuracy for owners who want precise control. They require proper calibration with buffer solutions and careful maintenance, but for serious hot tub enthusiasts, they’re worth the investment.
To obtain accurate results regardless of testing method, follow these best practices:
First, collect your water sample from elbow depth, away from jets and returns, where the water is most representative of the entire volume. Surface water or water near chemical injection points can give misleading readings.
Second, ensure the water is circulating but not actively aerating. Turn on the circulation pump but close the air valves and turn off the jets. Heavily aerated water can temporarily show falsely elevated pH due to CO2 off-gassing.
Third, test the water at normal operating temperature. Cold water and hot water hold different amounts of dissolved gases, which affects pH readings. If possible, test when the spa is at your typical soaking temperature of 100 to 104 degrees Fahrenheit.
Fourth, test multiple parameters simultaneously. Never test pH in isolation, always check Total Alkalinity, sanitizer level, and calcium hardness at the same time. These interrelated parameters provide the complete picture you need for informed decision-making.
Fifth, record your results in a log or app. Tracking trends over time helps you anticipate problems, identify patterns related to usage or weather, and fine-tune your maintenance routine.
Before adding any chemicals to increase pH in hot tub water, confirm that you’re addressing the right problem. If your test shows low pH but also reveals low alkalinity, you’ll need a different approach than if alkalinity is normal. This diagnostic step prevents wasted chemicals and potential overcorrection.
Chemical Solutions: Soda Ash vs. Baking Soda

The two primary chemicals for raising hot tub pH are sodium carbonate (soda ash) and sodium bicarbonate (baking soda). Understanding when to use each one is crucial for effective, stable water balance.
Sodium carbonate, marketed as “pH Up,” “pH Increaser,” or simply soda ash, is a powerful alkaline compound with a pH around 11.4. It’s specifically formulated to raise pH with minimal impact on Total Alkalinity. This makes it the ideal choice when your pH is low but your alkalinity is already within the proper range of 80 to 120 ppm.
When you add soda ash to water, it quickly dissociates into sodium ions and carbonate ions. The carbonate ions combine with hydrogen ions in the water, effectively removing acidity and raising pH. Because it’s so potent, you need relatively small amounts typically one to two tablespoons per 500 gallons to raise pH by 0.2 to 0.3 points.
Sodium bicarbonate, commonly known as baking soda or sold as “Alkalinity Increaser,” serves a dual purpose. It raises both Total Alkalinity and pH, though its impact on pH is more modest than soda ash. With a pH around 8.3, baking soda is the preferred choice when both your pH and alkalinity are low.
The chemical mechanism differs slightly. Sodium bicarbonate provides bicarbonate ions, which serve as the primary pH buffer in your water. These bicarbonate ions absorb both acids and bases, stabilizing pH against fluctuations. To achieve the same pH increase as soda ash, you’ll need approximately twice as much baking soda, but you’ll simultaneously strengthen your alkaline buffer.
Here’s a practical decision framework:
Use Soda Ash When:
- pH is below 7.2 AND Total Alkalinity is 80 ppm or higher
- You need to raise pH quickly without affecting alkalinity
- You’re fine-tuning pH after establishing proper alkalinity
Use Baking Soda When:
- Both pH and Total Alkalinity are below target ranges
- You’re establishing initial water balance in a freshly filled spa
- You need to strengthen the pH buffer to prevent future fluctuations
Cost Comparison and Practical Considerations
Many hot tub owners wonder whether generic household baking soda can substitute for branded spa chemicals. The answer is yes, with some caveats. Pure sodium bicarbonate is sodium bicarbonate, whether you buy it at the grocery store or the pool supply shop. Arm & Hammer baking soda works identically to a product labeled “Spa Alkalinity Up.”
However, sodium carbonate requires more caution. Pool-grade soda ash is pure sodium carbonate, but washing soda (sometimes confused with soda ash) may contain additives unsuitable for hot tubs. If you’re purchasing generic products, verify they’re pure, single-ingredient chemicals without fragrances, anti-caking agents, or other additives.
From a cost perspective, household baking soda typically costs 50 to 70 percent less than branded spa alkalinity increasers. For budget-conscious owners maintaining larger spas, this represents significant savings over time. Soda ash pricing is more competitive between generic and branded versions, with less dramatic price differences.
The branded spa products do offer some conveniences: pre-measured packaging, detailed usage instructions for spas specifically, and sometimes formulations that dissolve more readily. For new hot tub owners or those who prefer simplicity, these benefits may justify the premium price.
Regardless of which product you choose, proper storage is essential. Both chemicals absorb moisture from the air, forming clumps that reduce effectiveness. Store them in airtight containers in a cool, dry location away from incompatible chemicals like acids or oxidizers.
Step-by-Step Guide to Raising Hot Tub pH
Successfully raising your hot tub’s pH requires methodical execution and patience. Rushing the process or skipping steps often leads to overcorrection, cloudy water, or persistent instability. Follow this systematic approach for reliable, lasting results.
Safety Preparations
Before handling any spa chemicals, protect yourself with appropriate safety equipment. Wear chemical-resistant gloves to prevent skin irritation, and safety goggles to protect your eyes from splashes. Work in a well-ventilated area, especially when handling fine powders that can create dust.
Never mix chemicals together before adding them to water. The reactions can be violent, producing heat, toxic gases, or even explosions in extreme cases. Always add chemicals to water, never water to chemicals, to prevent concentrated reactions.
Step One: Balance Total Alkalinity First
This cannot be emphasized enough attempting to raise pH without first addressing low alkalinity is an exercise in frustration. Test your Total Alkalinity and bring it to 80 to 120 ppm before making pH adjustments.
If alkalinity is low, add sodium bicarbonate (baking soda) according to your water volume. A general guideline is one and a half tablespoons per 100 gallons to raise alkalinity by approximately 10 ppm. For a 400-gallon spa with alkalinity at 60 ppm that you want to bring to 100 ppm, you’d add about six tablespoons of baking soda.
Add the baking soda, circulate for at least 30 minutes, then wait four to six hours before retesting. This waiting period allows the alkalinity to stabilize. Once alkalinity is in range, you can address pH specifically.
Step Two: Calculate the Correct Dosage
With alkalinity properly established, determine how much pH increaser you need. The exact amount depends on your current pH, target pH, and water volume.
As a general starting point:
- To raise pH by 0.2 points in 400 gallons: add one tablespoon of soda ash
- To raise pH by 0.2 points in 400 gallons: add two tablespoons of baking soda (if using this instead)
Always start conservatively. It’s far easier to add more chemical after retesting than to correct an overshoot that pushes pH too high. For significant corrections (raising pH from 6.8 to 7.4, for example), plan to make the adjustment in two or three incremental additions over 24 hours.
Step Three: Dissolving the Chemicals
You have two primary methods for adding pH increaser to your spa: pre-dissolving in a bucket or broadcasting directly onto the water surface.
Pre-dissolving offers better control and faster integration. Fill a plastic bucket with one to two gallons of spa water, then slowly add the measured chemical while stirring with a plastic or wooden stick. Once fully dissolved, pour the solution around the perimeter of the spa, avoiding direct contact with the shell in any one spot.
Broadcasting involves sprinkling the dry chemical across the water surface while the circulation pump runs. The turbulence helps distribute and dissolve the powder. This method works well for small adjustments but can create temporary cloudiness if you add too much at once.
Never dump chemicals directly onto one spot, especially near the skimmer or on the spa shell. Concentrated chemicals can bleach or etch surfaces and damage equipment if they enter the plumbing system undissolved.
Step Four: Circulation and Mixing
After adding the pH increaser, run your circulation pump with jets on low for at least 15 to 20 minutes. Keep the air valves closed during this period the intense aeration can accelerate CO2 off-gassing and create artificially elevated pH readings that don’t reflect the true water chemistry.
The goal is thorough mixing without excessive turbulence. You want the chemical distributed evenly throughout all 400 or 500 gallons of water, reaching the far corners and bottom areas where circulation may be less vigorous.
Step Five: The Waiting Period
Patience is critical. While some chemical reactions occur quickly, the water needs time to reach a stable equilibrium state. Minimum waiting time is 30 minutes, but for more accurate readings, wait two to four hours before retesting.
During this period, avoid using the hot tub. Bather load will alter the chemistry before you can accurately assess the results of your adjustment. Keep the cover off or loosely positioned to allow gas exchange, which helps stabilize pH.
Temperature also affects readings, so if possible, retest when the water is at the same temperature as your initial test. A drop of even 10 degrees can shift pH readings by 0.1 to 0.2 points.
Step Six: Retesting and Adjusting
After the waiting period, test pH again using the same method you used initially for consistency. Compare the new reading to your target range of 7.4 to 7.6.
If pH is still below target, repeat the addition process with another conservative dose. Multiple small adjustments are always preferable to one large addition that overshoots the mark.
If pH is now within range, test the other parameters alkalinity, sanitizer, and calcium hardness to ensure everything remains balanced. Your alkalinity may have increased slightly even with soda ash, so verify it hasn’t climbed above 120 ppm.
If you’ve overshot and pH is now above 7.8, you’ll need to lower it using pH decreaser (sodium bisulfate or muriatic acid). For guidance on this process, see our detailed article on How to Lower Ph in Hot Tub.
Document your results, noting the product used, amount added, water volume, and time elapsed. This record becomes invaluable for future adjustments and helps you develop an intuitive understanding of your specific spa’s chemical behavior.
The Aeration Method: Raising pH Without Chemicals
For situations where your pH is low but Total Alkalinity is already at the high end of the acceptable range or even slightly elevated, adding more alkaline chemicals would push alkalinity too high. In these cases, aeration offers an elegant, chemical-free solution.
Aeration works by increasing the water’s contact with air, which promotes the off-gassing of dissolved carbon dioxide. Carbon dioxide in water forms carbonic acid, which lowers pH. By removing CO2, you reduce the acidity and allow pH to rise naturally.
The science behind this method is straightforward. When water is agitated and exposed to air, the concentration of dissolved CO2 equalizes with the atmospheric concentration through gas exchange. Since hot tub water typically contains more dissolved CO2 than atmospheric air, the net effect is CO2 leaving the water, which shifts the chemical equilibrium toward higher pH.
How to Implement the Aeration Method
Start by removing your spa cover completely to allow maximum air contact. Turn on all jets to their highest setting and fully open the air control valves. This creates maximum turbulence and bubbling, dramatically increasing the surface area where water meets air.
If your hot tub has a waterfall feature, activate it for additional aeration. Some models have dedicated air blowers run these as well to maximize the effect.
Maintain this high-aeration state for 30 minutes to two hours, depending on how much you need to raise the pH. Check the water every 30 minutes to monitor progress. You’ll typically see pH rise by 0.1 to 0.2 points per hour with aggressive aeration.
When to Use Aeration vs. Chemicals
Aeration is ideal when:
- pH is below 7.4 but Total Alkalinity is 110 ppm or higher
- You want to avoid adding more dissolved solids to the water
- You have time for the slower, gradual adjustment process
- You’re fine-tuning pH in the 7.2 to 7.4 range
Aeration is not suitable when:
- You need rapid pH correction due to bather comfort issues
- Total Alkalinity is also low and needs to be raised
- Your pH is severely depressed (below 7.0)
- The environmental temperature is very cold, which slows gas exchange
Advantages and Limitations
The primary advantage is that aeration adds nothing to the water, avoiding the incremental buildup of Total Dissolved Solids that comes with chemical additions. It’s also free, using only electricity to run the pumps and blowers you’d use for regular soaking anyway.
The main limitation is time. Aeration raises pH much more slowly than chemical additives. If you’re dealing with uncomfortable, acidic water and guests arriving in an hour, chemicals are the practical choice. Aeration is best for routine fine-tuning and situations where you have the luxury of letting the process work gradually.
Noise is another consideration. Running jets at maximum with air valves fully open creates significant sound that may disturb neighbors or family members if you’re doing this in the evening. Energy consumption also increases during extended high-speed operation.
Some hot tub owners incorporate brief aeration periods into their regular maintenance routine running jets fully open for 15 to 20 minutes weekly as a preventive measure to avoid pH drift downward. This proactive approach often reduces the frequency of chemical adjustments.
Managing pH in Different Hot Tub Types

Not all hot tubs are created equal when it comes to water chemistry management. The construction materials, volume, filtration systems, and even the heat source affect how you should approach pH correction and maintenance.
Acrylic Spas
Traditional acrylic hot tubs represent the standard for residential installations. These units feature durable acrylic shells reinforced with fiberglass, sophisticated filtration and heating systems, and typically hold 300 to 500 gallons of water.
For acrylic spas, the pH adjustment process follows the standard guidelines outlined in previous sections. The shell material is chemically inert and unaffected by the slight pH fluctuations that occur during correction. The plumbing and equipment are designed to handle chemical additions when properly diluted and distributed.
One consideration specific to acrylic tubs is the ozonator if your model includes one. Ozone generators produce ozone gas that helps sanitize water but also gradually lowers pH over time as the ozone breaks down into oxygen and other compounds. If you have an ozonator, you may find yourself raising pH more frequently than hot tub owners without this feature.
Inflatable Hot Tubs
Inflatable spas have surged in popularity due to their affordability and portability, but their vinyl construction requires extra care during pH adjustment. The vinyl liner can be sensitive to concentrated chemicals, developing brittle spots or discoloration if harsh chemicals contact the material directly.
When raising pH in an inflatable hot tub, always pre-dissolve chemicals completely in a bucket before adding them to the spa. Never broadcast dry powder onto the water surface or pour concentrated solutions near the walls.
Inflatable models also typically have smaller water volumes often 200 to 300 gallons which means you’ll need proportionally less chemical. A dose appropriate for a 500-gallon acrylic spa would significantly overcorrect a 200-gallon inflatable unit. Scale your measurements carefully based on actual water volume, not estimates.
The filtration systems in inflatable tubs are generally less powerful than those in acrylic spas, so allow extra time for chemical distribution. Run the filter for 30 to 45 minutes after adding pH increaser to ensure thorough mixing.
Saltwater Hot Tubs
Saltwater systems use electrolysis to convert dissolved salt into chlorine for sanitization. This process has profound effects on pH behavior. The electrolysis reaction is inherently alkaline-producing, which means saltwater hot tubs typically experience upward pH drift rather than the downward drift common in traditional systems.
However, saltwater spas still occasionally need pH raised, particularly after fresh fills or when heavy bather loads temporarily suppress pH. The adjustment process is the same as standard hot tubs, but monitor closely after correction the salt cell will likely push pH upward naturally over the following days.
Consider reducing salt cell output slightly if you find yourself constantly lowering pH. The system may be producing sanitizer faster than your actual usage requires, creating excess pH elevation as a side effect.
Wood-Fired Hot Tubs
Traditional wood-fired hot tubs, often called Japanese soaking tubs or Norwegian hot tubs, present unique challenges. These units lack circulation pumps and filtration systems, relying on convection currents created by the wood-fired heater to move water.
Without mechanical circulation, chemical distribution is much slower and less uniform. When raising pH in a wood-fired tub, pre-dissolve the chemical completely, then pour it slowly around the entire perimeter of the tub. Stir the water manually with a clean paddle or stick to promote mixing.
Allow at least four to six hours before retesting, and sample water from multiple locations to ensure readings are consistent throughout the volume. The lack of filtration also means these tubs benefit from more frequent water changes often weekly rather than quarterly which provides regular opportunities to reset water chemistry from scratch.
Wood-fired tubs often use cedar or other aromatic woods that slowly release tannins and oils into the water, creating a natural tendency toward lower pH. Plan for more frequent pH testing and adjustment than you’d need with conventional spas.
Troubleshooting Common pH Problems
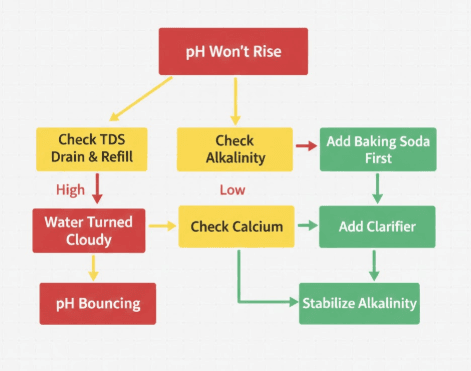
Even when you follow procedures carefully, pH management sometimes presents stubborn challenges. Understanding these common scenarios and their solutions will save you frustration and wasted chemicals.
Scenario A: The Unchanging pH (pH Lock)
You’ve added pH increaser according to guidelines, waited the recommended time, retested, and discovered the pH hasn’t budged. You add more chemical with the same disappointing result. This frustrating situation indicates a condition sometimes called “pH lock” or severely aged water.
The root cause is typically excessive Total Dissolved Solids. After months without a water change, your spa water accumulates dissolved minerals, spent chemicals, organic compounds, and other substances. When TDS exceeds 1,500 to 2,000 ppm, the water becomes saturated and resistant to chemical adjustments.
Test your TDS using a digital TDS meter. If readings exceed 1,500 ppm, no amount of pH increaser will provide stable correction. The solution is to drain the spa completely, clean the shell thoroughly, and refill with fresh water. This “chemical reset” restores your ability to manage pH effectively.
Before draining, note your source water chemistry. If your tap water has very low alkalinity (below 50 ppm), you’ll need to establish proper alkalinity immediately after filling to create a stable foundation.
Another potential cause is interference from metals, particularly copper or iron. These metals can complex with alkalinity buffers, effectively neutralizing them. If you have metal staining or green water discoloration, use a metal sequestering agent before attempting further pH adjustments.
Scenario B: Cloudy Water After pH Correction
You successfully raised pH into the proper range, but within hours the water became cloudy or milky. This indicates calcium carbonate precipitation, commonly called “calcium fallout” or scaling.
The problem stems from the relationship between pH, calcium hardness, and Total Alkalinity. At higher pH levels, calcium becomes less soluble and precipitates out of solution as tiny white particles that cloud the water. If your calcium hardness is above 250 ppm and you rapidly increase pH, you create conditions favorable for this precipitation.
Test calcium hardness immediately. If it’s elevated, you have two options. First, you can lower pH slightly back into the 7.2 to 7.4 range where calcium is more soluble, though this partially undoes your correction. Second, you can add a sequestering agent or scale inhibitor that keeps calcium in solution even at higher pH levels.
For immediate clearing, add a water clarifier designed for spas. These products coagulate the fine calcium particles into larger clumps that the filter can capture. Run the filtration system continuously for 24 to 48 hours, cleaning or replacing the filter cartridge as it captures the precipitated calcium.
Prevention is preferable to cure. Before raising pH in water with high calcium levels, add a scale defense product. These preventive treatments are much more effective than trying to reverse precipitation after it occurs.
Scenario C: Wild pH Swings (pH Bounce)
Your pH readings are erratic and unpredictable 7.0 one day, 7.8 two days later, then back down to 7.2. This “pH bounce” makes it impossible to maintain stable water chemistry and indicates inadequate alkalinity buffering.
Despite what your most recent test showed, retest Total Alkalinity carefully. If it’s below 80 ppm, the root cause is confirmed. Low alkalinity provides insufficient buffering capacity to resist pH changes from bather load, chemical additions, and environmental factors.
The solution is to raise and stabilize alkalinity first, targeting 100 ppm. Add sodium bicarbonate (baking soda) according to your water volume, circulate thoroughly, wait six hours, and retest. Once alkalinity stabilizes in the 90 to 110 ppm range, pH swings will diminish dramatically.
If alkalinity tests in the proper range but pH still bounces, consider your sanitizer type and dosing frequency. Trichlor tablets or bromine tablets are acidic and suppress pH with each dose. If you’re adding these daily, you’re creating a daily acid shock that overwhelms your alkaline buffer. Switch to a less acidic sanitizer like liquid chlorine (sodium hypochlorite) or reduce dosing frequency.
Scenario D: Green Water After Chemical Addition
After adding pH increaser, your water took on a green, blue, or brown tint. This alarming color change indicates the presence of dissolved metals copper producing green or blue, iron producing brown or rust colors.
Metals enter hot tub water through source water (especially well water), corroded equipment, or certain algaecides containing copper compounds. At low pH, these metals remain dissolved and invisible. When you raise pH, the metals precipitate out of solution and become visible as colored staining or water discoloration.
Immediate action is required to prevent permanent staining of your spa shell. Add a metal sequestering agent immediately products like Metal Gone, Metal Free, or similar chelating agents. These chemicals bind to metal ions and hold them in solution, preventing them from depositing on surfaces.
Run the filtration continuously for 48 hours. The sequestered metals will partially bind to the filter media. Clean or replace your filter cartridge after this treatment.
For long-term prevention, test your source water for metals before filling. If copper or iron levels are detectable, add a metal sequestering agent to the fresh water before heating the spa. Maintain the sequestrant with regular additions according to the product label typically monthly.
Consider installing a pre-filter on your hose when filling the spa. These specialized filters remove metals, minerals, and other contaminants from source water, providing a cleaner starting point for chemical balance.
Prevention and Long-Term Maintenance
Effective hot tub ownership isn’t about constantly reacting to water chemistry crises it’s about establishing routines that prevent problems before they develop. Strategic prevention reduces chemical usage, extends equipment life, and ensures your spa is always ready for relaxation.
Establish a Consistent Testing Schedule
The foundation of prevention is knowledge. Test your water at least twice weekly, even when the spa appears perfectly clear and comfortable. pH can drift significantly in just 72 to 96 hours, especially with regular use.
Develop a testing routine tied to specific days Tuesday and Saturday mornings, for example so it becomes automatic rather than something you remember to do sporadically. Keep your test kit or strips in a visible location as a reminder.
Record every test result in a notebook or smartphone app. This historical data reveals patterns specific to your spa. You might discover that pH drops consistently three days after shocking, or that heavy weekend use requires a midweek pH boost. These insights allow you to anticipate needs rather than simply responding to problems.
Implement Proactive Alkalinity Management
Since Total Alkalinity is the foundation of pH stability, monitor it carefully and maintain it at the optimal 100 ppm level. When alkalinity drifts toward the lower end of the acceptable range (80 to 90 ppm), proactively raise it back to 100 ppm rather than waiting for it to drop further.
This small, preventive addition of baking soda costs little and prevents the larger pH problems that develop when alkalinity becomes insufficient. Think of it as routine maintenance like checking tire pressure a small effort that prevents bigger issues.
Choose Sanitizers Strategically
Your sanitizer choice has profound implications for pH behavior. Trichlor chlorine tablets (pH around 3.0) constantly suppress pH and require frequent correction. Bromine tablets (pH around 4.0) have a similar effect, though slightly less severe.
Liquid chlorine (sodium hypochlorite) has a pH of 13.0, actually raising pH slightly with each dose. For spas that tend toward low pH, switching to liquid chlorine can reduce or eliminate the need for pH increaser. The trade-off is that liquid chlorine has a shorter shelf life and requires more frequent application since it doesn’t dissolve slowly like tablets.
Dichlor granular chlorine (sodium dichloro-s-triazinetrione) is nearly neutral with a pH around 6.9 and offers a balanced option that minimally affects pH. Many hot tub professionals consider it the ideal sanitizer for residential spas.
Non-chlorine shock (potassium monopersulfate) is slightly acidic but far less so than chlorine or bromine tablets. Using non-chlorine shock weekly instead of chlorine shock reduces cumulative pH suppression.
Manage Bather Load Effectively
Every person who uses your hot tub introduces contaminants that affect water chemistry. While you can’t eliminate bather impact, you can manage it intelligently.
Require all bathers to shower before entering the spa, rinsing off body oils, lotions, deodorants, hair products, and other cosmetics that contaminate water and consume sanitizer. This simple rule can reduce chemical demand by 30 to 50 percent.
After parties or sessions with multiple guests, shock the spa that evening or the next morning. This oxidizes the organic waste before it has time to decompose and acidify the water. Proactive shocking prevents the pH crash that would otherwise occur 24 to 48 hours after heavy use.
Consider implementing a “bathing suit only” rule no street clothes in the spa. Regular clothing contains detergents, fabric softeners, and dyes that introduce phosphates and other chemistry-disrupting compounds.
Regular Water Changes
No amount of chemical management can compensate indefinitely for water that has simply aged beyond usefulness. Plan for complete water changes every three to four months under normal usage conditions, or more frequently with heavy use.
Fresh water provides a chemical reset, eliminating accumulated TDS, spent chemicals, and dissolved organics that make water increasingly difficult to balance. Many experienced hot tub owners schedule water changes seasonally fall, winter, spring, and summer making it an automatic part of the ownership routine.
During the drain-and-refill process, clean the spa shell thoroughly, flush the plumbing lines with a specialized cleaner, and clean or replace filter cartridges. This comprehensive reset extends equipment life and makes subsequent chemical management dramatically easier.
Protect Against Environmental Contamination
Keep your spa covered when not in use. A quality, properly fitted cover prevents rain (which can be acidic) from entering the water, keeps out leaves and debris, reduces evaporation that concentrates dissolved solids, and minimizes dust and pollen contamination.
In regions with acid rain concerns, covering becomes even more critical. Acid rain with pH as low as 4.0 can dilute and acidify hundreds of gallons of spa water surprisingly quickly during a heavy storm.
Trim back overhanging trees and shrubs that drop leaves, flowers, seeds, or pollen into the spa. These organic materials decompose and acidify water while consuming sanitizer.
Use Sequestering Agents for Problem Water
If your source water contains metals or high mineral content, add a sequestering agent immediately after every fill and maintain it with monthly additions. These products are inexpensive insurance against metal staining and mineral scaling, both of which complicate pH management.
Metal-free water is far easier to balance and maintain than water with dissolved copper or iron. The modest cost of sequestrants typically ten to fifteen dollars per bottle lasting several months is easily justified by the problems they prevent.
By implementing these prevention strategies, you’ll find that maintaining proper pH becomes routine rather than challenging. The spa that once required weekly chemical corrections may need only occasional fine-tuning, giving you more time to enjoy the relaxation you bought the hot tub for in the first place.
Frequently Asked Questions About Raising Hot Tub pH
Can I use baking soda to raise pH in my hot tub?
Yes, baking soda (sodium bicarbonate) effectively raises both pH and Total Alkalinity in hot tubs. It’s particularly useful when both parameters are low, providing a double benefit. However, if your alkalinity is already in the proper range and only pH needs adjustment, soda ash (pH Up) is the better choice since it raises pH with minimal impact on alkalinity.
Household baking soda works identically to branded “Alkalinity Up” products and costs significantly less. Use one and a half tablespoons per 100 gallons to raise alkalinity by approximately 10 ppm, which will also increase pH by 0.1 to 0.2 points.
How long do I have to wait to get in the hot tub after adding pH increaser?
Wait at least 20 to 30 minutes after adding pH increaser before entering the hot tub. This allows time for the chemical to distribute evenly throughout the water and for the pH to stabilize. For more accurate assessment of the final pH level, waiting two to four hours is ideal before retesting.
If you’ve made a large correction or added substantial amounts of chemical, extending the wait to one to two hours ensures complete mixing and reduces the risk of localized chemical concentration that could cause skin irritation.
Is it safe to use a hot tub with low pH?
Using a hot tub with low pH is not recommended and can be unsafe. Acidic water causes eye irritation, skin discomfort, and respiratory issues from increased chloramine formation. Beyond immediate discomfort, low pH corrodes metal components in heaters and pumps, potentially causing equipment failure or releasing metals into the water.
If pH is only slightly low (7.0 to 7.2), brief use while you’re addressing the problem is probably acceptable, though not ideal. If pH has dropped below 7.0, avoid using the spa until you’ve corrected the imbalance. The corrosion risk to equipment and potential for bather discomfort outweigh the temporary enjoyment.
What is the difference between pH Up and Alkalinity Up?
pH Up (soda ash or sodium carbonate) primarily raises pH with minimal effect on Total Alkalinity, while Alkalinity Up (baking soda or sodium bicarbonate) raises both alkalinity and pH together. The key difference lies in their chemical composition and how they affect water buffering capacity.
Use pH Up when your alkalinity is already balanced but pH remains low. Use Alkalinity Up when both parameters need adjustment or when you want to strengthen the pH buffer to prevent future fluctuations. Understanding this distinction prevents overcorrecting one parameter while fixing another.
Why does my hot tub pH keep dropping?
Persistent pH decline typically results from low Total Alkalinity, which provides insufficient buffering against acidifying factors. Other common causes include acidic sanitizers like trichlor or bromine tablets, heavy bather load introducing organic acids, environmental contamination from acid rain, and source water with naturally low pH or alkalinity.
To solve recurring pH problems, first ensure Total Alkalinity is maintained at 100 ppm. Then evaluate your sanitizer choice switching from acidic tablets to dichlor granules or liquid chlorine often eliminates chronic pH suppression. Finally, implement prevention strategies like requiring pre-soak showers and keeping the spa covered when not in use.
Can I use vinegar to raise hot tub pH?
No, vinegar cannot raise pH it actually lowers pH because it’s acidic. Vinegar (acetic acid) has a pH around 2.5 to 3.0 and would make low pH problems worse. This common misconception likely stems from confusion with using vinegar to lower pH in certain applications.
To raise pH, you need alkaline substances like sodium carbonate (soda ash), sodium bicarbonate (baking soda), or specialized pH increaser products. Never add acidic substances when trying to increase pH.
How much pH increaser should I add to a 400-gallon hot tub?
For a 400-gallon hot tub, add approximately one to two tablespoons of soda ash (pH Up) to raise pH by 0.2 to 0.3 points. If using baking soda instead, double that amount to three to four tablespoons for similar results. These are general guidelines always start with the conservative amount, test after 30 minutes to two hours, and add more if needed.
Your specific water chemistry, current pH level, and Total Alkalinity affect the exact amount required. Keeping detailed records of your adjustments helps you develop precise dosing for your particular spa over time.



